Your Surgeon’s Choice of Breast Implant
I help my patients understand the choices available and will often make recommendations on the most suitable products. I find the Mentor breast implant range offer a very good choice for patients. Although it is not ideal in every situation and there are alternatives, I have a detailed knowledge of the pros and cons of using different implants in different situations, and patients get the benefit of over 16 years of experience that I have in using these products. Through this expertise I produce optimal results with breast enlargement surgery.
Your Choice of Breast Implant
When choosing a breast implant you need to consider safety, longevity, and how it will serve its purpose in your individual situation. Mentor MemoryGel and MemoryGel Xtra Breast Implants are used in both cosmetic breast surgery for breast enlargement and for breast reconstruction. This is a global brand with an established track record. Mentor implants are made in the USA and are both FDA and MHRA (European CE mark) approved, and their implants have been in use for over 30 years. In comparison to saline implants these feel more natural, more like real breast tissue.
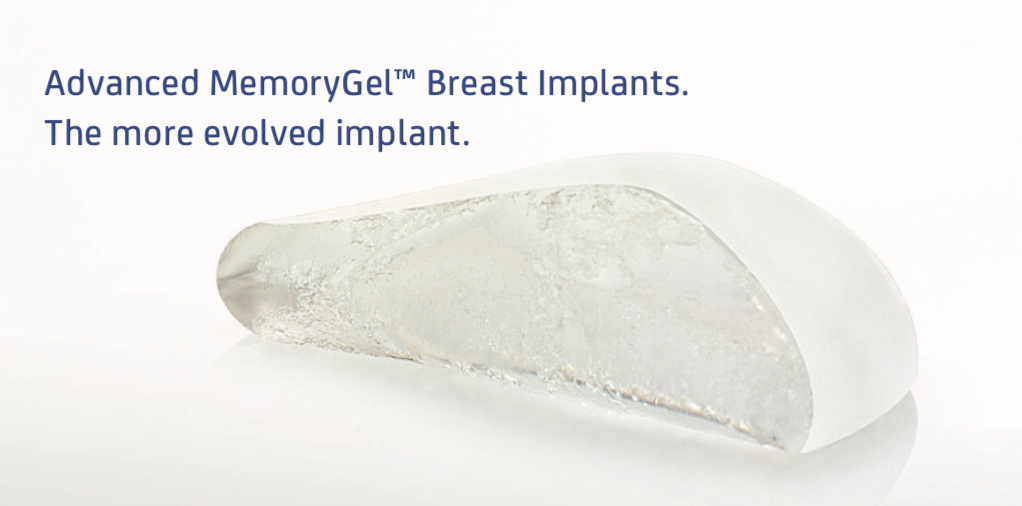
Cutting the Mentor MemoryGel Breast implant in half reveals the highly cohesive internal gel
- FDA approved
- Filled with Mentor’s proprietary cohesive gel
- Gel filler holds together uniformly to look and feel like natural breast tissue
- Set fill volume for predictable results
- Six projection options: classic, moderate plus, high profile and ultra high profile (MemoryGel®)
moderate plus and high profile (MemoryGel® Xtra) - Two shell surface options: smooth or textured (the texturing is not aggressive, and commonly referred to as micro texture)
- Covered by the Mentor Warranty and Mentor Enhanced Warranty
View the Mentor_Implant_Product_Catalogue_2021
View the Old Mentor Catalogue
All MENTOR® Silicone Gel-Filled Breast Implants contain gel that is cohesive, conforms to safety standards and aesthetically pleasing. There are three different levels of cohesive gel availalable allowing your surgeon some extra flexibility in matching the implant to your needs.
Cohesive one
This is the standard cohesive level gel used in MENTOR® Breast Implants. This is the softest gel and has been preferred for years in textured and smooth Round Low, Moderate, Moderate Plus, High, and Ultra High Profile Gel Breast Implants as well as the BECKERTM Expander/ Breast Implants.
Cohesive two
This is a slightly firmer gel, and I find this useful under tighter tissue, and where I want to impart the shape of the implant more on the surrounding breast. This gel is used in the entire textured Round Moderate, Moderate Plus, High Profile Gel Breast Implants and Becker Expanders.
Cohesive three
This is Mentor’s most cohesive gel. It is a firmer implant and this serves a specific purpose in the tear drop implants to ensure shape retention with a pleasing level of firmness for optimal aesthetic results with tear drop implants. This gel is used only in the MENTOR CPG Gel Breast Implants.
Limited Mentor Breast Implant Warranty
The Mentor breast implant warranty is provide by Mentor. There is free automatic enrolment. The warranty is essentially that there is a lifetime product replacement in case of confirmed rupture. This means Mentor will provide your surgeon with the replacement breast implant free of charge. You remain responsible for the usual costs associated with surgery.
• In the case of a confirmed rupture, Mentor will also provide up to €1,000 of financial assistance, for a period of 10 years from the date of its implantation.
• Free product replacement in the case of confirmed capsular contracture Baker grade III and IV in augmentation surgery, for a period of 10 years from the date of its implantation.
Mentor’s Pledge
Mentor is a global leader in aesthetic medicine and was founded in 1969. Mentor Worldwide LLC is a leading supplier of medical products for the global aesthetic medicine market.
- Our primary concern is always for the health and safety of our patients.
- Our product innovation reflects patient and marketplace needs around the world.
- Our gel-filled breast implant products are manufactured with medical grade silicone, in regulated facilities and are designed to meet all relevant quality, safety and manufacturing standards while undergoing rigorous testing.
- As one of only four manufacturers with U.S. Food & Drug Administration (FDA) approved breast implants sold in the United States, we are committed to maintaining vigilant reporting procedures to continuously monitor the safe and effective use of our products while working in active partnership with the FDA and global regulatory authorities.
- We offer professional education to the health care community to ensure that our products are utilized correctly and in a compliant manner.
At Mentor, we are committed to making safe products that offer high quality, reliability and state-of-the-art design to enhance aesthetic and reconstructive procedures.
Safety
Mentor’s core study safety data showed just 3.8% capsular contraction rate at 10 years with the CPG Mentor Implants. The anatomical implants had a 1.5% rotation rate in this study. It is noteworthy that in a series of 9000 Siltex textured Mentor breast implants, there was not a single case of complication in the form of double capsule formation.
Rare Health Conditions
A recent study claiming silicone breast implants were associated with a higher risk of some rare health conditions was published in the Annals of Surgery. Science and long-term data from multiple clinical studies, including two 10-year prospective clinical trials, support the safety and clinical performance of breast implants. The FDA has stated that they respectfully disagree with the author’s conclusions. Along with the presidents-elect of both the American Society of Aesthetic Plastic Surgery and Plastic Surgery Foundation, and surgeons from Harvard Medical School and Memorial Sloan Kettering Cancer Center, the FDA also warned patients and surgeons to view the assessment with caution.
The conclusions in the article rely on an analysis of study data with significant flaws that have been acknowledged by FDA, unbiased medical experts and breast implant manufacturers. In fact, the article uses data from original studies that were replaced with redesigned studies because they were inadequate to determine associations with the rare health effects outlined in the article.
Mentor fully supports ongoing research and continues to partner with independent groups and the FDA to further scientific evidence related to breast implant safety. We share updated product safety information with surgeons in our product labeling, with patients in brochures, and online as appropriate. There are inherent risks associated with breast implants and MENTOR warns of such known risks in its Product Insert Data Sheets (PIDS) provided to surgeons and patient brochures provided to patients.
It is important for every woman considering breast implants to review all this information with her surgeon.
Click here to view the FDA’s full statement on the manuscript published in the Annals of Surgery.
Important Safety Information
Important information for women considering breast augmentation:
Important information for women considering breast reconstruction:
Important information for women considering breast augmentation or reconstruction:
BREAST-IMPLANT ASSOCIATED ANAPLASTIC LARGE CELL LYMPHOMA (BIA-ALCL)
Mentor continues to work with industry groups, physician scientists and health authorities globally to better understand the associated risks and causes of BIA-ALCL. As patient safety has been and always will be Mentor’s first priority, Mentor continues to closely monitor reports of and information about BIA-ALCL.
MENTOR MemoryShape Breast Implants
MENTOR MemoryShape Breast Implants are indicated for breast augmentation in women at least 22 years old, or for breast reconstruction. Breast implant surgery should not be performed in women with active infection anywhere in their body, with existing cancer or pre-cancer of their breast who have not received adequate treatment for those conditions, or who are pregnant or nursing.
Breast implants are not lifetime devices and breast implantation may not be a one-time surgery. The most common complications with MENTOR MemoryShape Breast Implants for breast augmentation include reoperation for any reason, implant removal with or without replacement, and ptosis. The most common complications with MENTOR MemoryShape Breast Implants for breast reconstruction include reoperation for any reason, implant removal with or without replacement, and capsular contracture. A lower risk complication is rupture. The health consequences of a ruptured silicone gel breast implant have not been fully established. MRI screenings are recommended three years after initial implant surgery and then every two years after to detect silent rupture.
Patients should receive a copy of Patient Educational Brochure – Breast Augmentation with MENTOR® MemoryShape® Breast Implants or Patient Educational Brochure – Breast Reconstruction with MENTOR® MemoryShape Breast Implants, and a copy of Quick Facts about Breast Augmentation & Reconstruction with MENTOR MemoryShape Breast Implants. Your patient needs to read and understand the information regarding the risks and benefits of breast implants, with an opportunity to consult with you prior to deciding on surgery.
For detailed indications, contraindications, warnings, and precautions associated with the use of MENTOR MemoryShape Breast Implants please refer to the Product Insert Data Sheet provided with each product, or visit www.mentorwwllc.com.
MemoryGel Breast Implants
MemoryGel Breast Implants are indicated for breast augmentation in women at least 22 years old or for breast reconstruction. Breast implant surgery should not be performed in women with active infection anywhere in their body with existing cancer or pre-cancer of their breast who have not received adequate treatment for those conditions or are pregnant or nursing.
Breast implants are not lifetime devices and breast implantation is not necessarily a one-time surgery. The most common complications with the MemoryGel®Breast Implants include reoperation, capsular contracture, asymmetry, and breast pain. A lower risk of complication is rupture. The health consequences of a ruptured silicone gel-filled breast implant have not been fully established. MRI screenings are recommended three years after initial implant surgery and then every two years after to detect silent rupture.
Patients should receive a copy of Important Information for Augmentation Patients about MENTOR MemoryGel Silicone Gel-Filled Breast Implants or Important Information for Reconstruction Patients about MENTOR MemoryGel Silicone Gel-Filled Breast Implants. Your patient needs to read and understand the information regarding the risks and benefits of breast implants, with an opportunity to consult with you prior to deciding on surgery.
For detailed indications, contraindications, warning and precautions associated with the use of MemoryGel® Breast Implants please refer to the Product Insert Data Sheet provided with each product, or online at www.mentorwwllc.com.

